Applying Lean Thinking in Clinical Pathology – Application in Histocompatibility and Immunogenetics at NHS Blood and Transplant
Background
Since 2008, the UK’s NHS Blood and Transplant (NHSBT) has been successful in using lean tools to become a more effective and efficient service and from 2013 the approach has been used to drive improvements in the Histocompatibility and Immunogenetics (H&I) function.
The focus of the H&I work was a national Value Steam Analysis (VSA) of the laboratory network, which consists of six laboratories across England. VSA is performed annually to focus CI activity and one outcome from the initial national VSA identified that changes in laboratory accreditation standards required the addition of a purity assessment method in the Chimerism service. Chimerism is a valuable tool in the assessment of engraftment and graft failure post-Haematopoietic cell transplant.
It was shown that a new cell separation method was required, as it was not possible to adhere to the new accreditation standards with the existing method. An alternate method for cell separation was evaluated, validated and implemented (by established NHSBT change management process).
The newly implemented method proved to be more involved than that previously employed method from comparison of the flow, touch and Takt times. At this time a steady 20% increase in annual workload was observed, with no additional staff resource was available and the method was associated with a sample repeat rate of greater than 60%.
Getting Started
Adapting to change
A chimerism value stream was created (figure 1) which prompted a Rapid Improvement Event (RIE) involving staff of all grades from multiple laboratories. Through the use of lean tools (including A3, 8 wastes, process mapping, gap analysis and standard work) a plan for short and long term objectives for Chimerism was agreed in order to adhere to good practice guidelines and accreditation standards.
The RIE was held over five days and led by a colleague who was specifically chosen due to no direct involvement in the chimerism process. The team consisted of representative staff from all grades performing the process across the NHSBT H&I network. This team was joined by an in-house CI representative, a ‘fresh pair of eyes’ from another pathology speciality and a member of the Quality Assurance department.
Standard work was established for each cell and potential bottlenecks were identified (automated extraction platform and genetic analyser due to run capacity). As a result, optimal batch sizes for each cell was agreed to work towards a pull system.
Figure 1 – Chimerism Value Stream
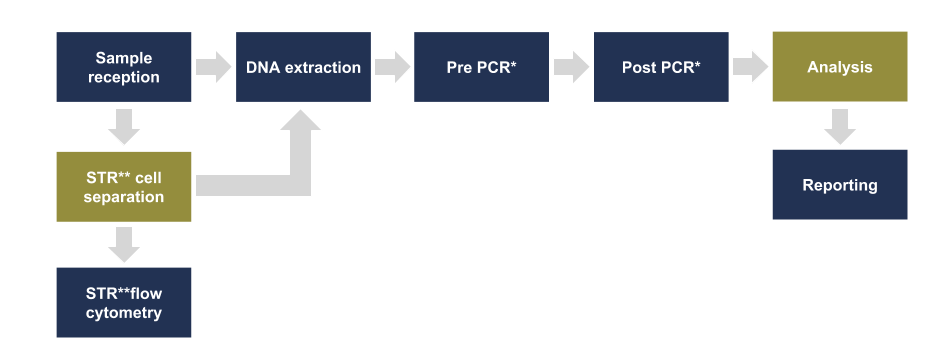
Figure 1: An overview of the cells required in the chimerism value stream. The cells highlighted in orange are exclusive to the chimerism process. All the remaining cells, highlighted in blue, accommodate multiple values streams.
*Polymerase Chain Reaction **Short Tandem Repeat
Removal of waste
Review of the value and non-value added steps identified improvements to the process that could be made immediately – the ‘quick wins’. For example, all of the patient data was stored in increasing large paper files, which were just duplications of raw data and analysis already held in electronic format. Organisation of the local shared storage (backed up on network) allowed easy access to historical data. As a result, each paper file was discarded in preference of an electronic folder for each patient.
Addressing the repeat rate
Standard work provided the platform by which the variability in the process was removed. This included standardising the DNA concentration, optimising the polymerase chain reaction process and creating a standard approach to analysis
Reagent review
A review of other known issues in the process identified that problems consistently arose with supply from commercial companies for key reagents. Where possible these have been replaced with validated in-house alternatives.
Semi-automated analysis
A commercial software package was validated for routine use to reduce potential transcription errors. It also allowed user audit and individual access levels to be set while also providing a standard analysis for all transplant recipients regardless of operator. As an added bonus, statistical analysis and trending of results was more readily available for ISO15189 compliance.
Review of cell separation
A steady increase in workload had pushed the cell separation method to capacity. No immediate alternative was available, but after consultation with suppliers a new robust method was created in collaboration with a third party by adaptation of their existing repertoire. This new method has been validated and implemented across the network.
Study & Measurement of the Lean Improvements
The primary impacts of the improvements on the chimerism service were measured by laboratory Key Performance Indicators (KPIs). KPIs include the number of referrals received, the mean sample turnaround time (TAT) from receipt in the laboratory to clinical report being issued and how many samples failed to meet service level agreed targets (not reported within 5 working days of receipt). These KPIs also contribute to the overall performance of the laboratory, which is openly reported on a monthly and annual basis.
As each improvement has been implemented, standard work has been reviewed to optimise performance. Performance of the chimerism service is trended (KPIs, mean TAT, repeat rates) and discussed in monthly laboratory section reports. Trends have been incorporated into a visual management display.
Results
CI – What does it look like?
Since the initial RIE and a need to address shortcomings in the chimerism process, continuous improvements have been suggested, reviewed and implemented over the last five years (figure 2).
Figure 2 – Time line of interventions in the chimerism process

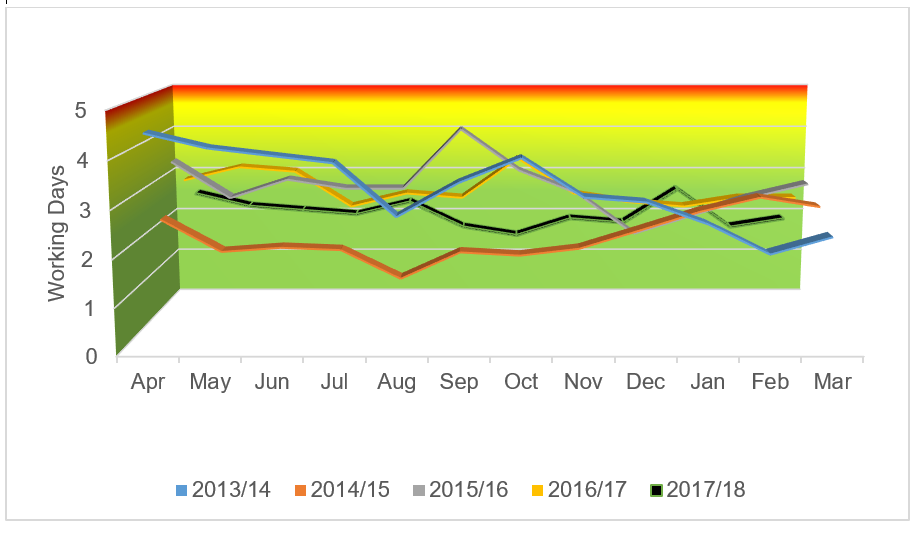
Over the five years that CI has been actively applied to this process, an increased sample workload has been observed (figure 3), while at the same time sample TAT has remained within both service level agreements and recommendations from the best practice guidelines (figure 4).
Figure 3 – Total number of chimerism DNA samples analysed annually
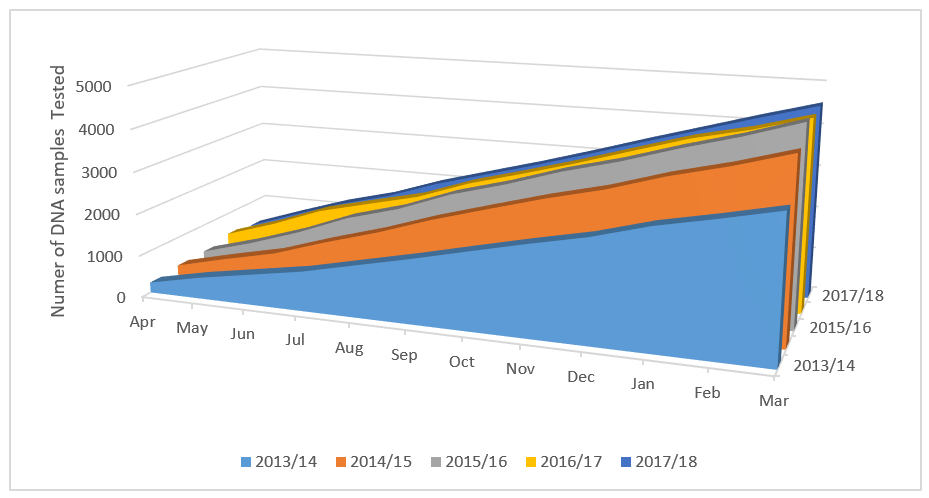
Figure 4 – Mean chimerism sample turnaround (TAT)
Applying CI in the real world
Since CI has been adopted in the laboratory there has been unintended contextual change within the laboratory service, including new equipment, changing/overlapping process value streams, as well as change to the footprint and layout of the laboratory. All of these events have had an impact on standard work.
By utilising CI during these changes the laboratory has observed positive outcomes, such as increased capacity (e.g. more suitable equipment, management its use) and less waste (e.g. motion due to a smaller footprint). CI activity in chimerism has impacted other parallel value streams that utilise shared cells. In these situations, all affected processes have been considered when optimising both standard work and batch sizes.
Counting the £’s?
The costings in the chimerism process have been reviewed at the end of each financial year throughout these improvements (inclusive of staff time, reagents, asset maintenance and software licencing). They have shown the cost per sample has remained consistent allowing the chimerism service to remain financially viable without additional cost to the customer/user, which is in-keeping with NHSBT financial planning.
By implementing standard work, extra capacity has been created within the process to accommodate a potential 70% increase on current workload. This has been achieved with no increase in staffing provision or running costs.
Discussion
Summary
Continual improvement and optimisation of the chimerism method has produced a high quality, low cost, rapid throughput clinical service. Standard work has been defined, KPIs are compliant and positive feedback has been received from both laboratory staff and users. One service user has even lodged an official appreciation of the current service provision through the hospital liaison compliment pathway.
Attention has also been given to the human dimension and small adjustments have been continually suggested and tried based on staff feedback, as well as more major changes such as change of cell separation technique (implementation of alternative technique), modification/optimisation of cell composition/layout (e.g. set-up/orientation and location of cell separation) and work flow within multi-functional cells (e.g. use of flow cytometer and genetic analyser).
Observed Limitations
The irregular work flow due to the user clinic times, the associated time limits of sample processing time given in the best practice guidelines and perceived clinical urgency have, as yet, prevented the process from being a true ‘pull’ system. To date there have been ongoing discussions with the service users to move the clinics and even workflow.
Stumbling blocks and learning points
At the start of the improvement process the CI and lean methodologies were not very well cascaded and were applied in a top-down fashion. The resulting lack of awareness and explanation to justify early interventions created an air of unrest and wariness. After the initial RIE there was also resistance to further CI activity in the process, as to many both in the laboratory and in directorate management, it was viewed as ‘we have already done that process’.
Parallel, stand-alone RIEs targeting individual laboratory cells that accommodate multiple process value streams have impacted and often hindered improvements and standard work already implemented.
These early issues have been largely overcome by involvement of all staff in the CI process and focus on the practical application, where the benefits can be seen day-to-day, not only on A3’s and spreadsheets. The interaction/overlap of value streams has been addressed by including all stakeholders for comments feedback throughout CI activity, from planning to implementation.
Finally, organisational restrictions within NHSBT, due to the structure and legality of financing and tendering within NHSBT, the potential of CI tools such as PDSA is yet to be fully realised. For instance, improvements that affect the testing process cannot only be put into place locally due to potential for non-compliance with TPS51/ISO15189 and significant process deviations/alterations could require reassessment laboratory accreditation bodies.
Author’s final thoughts & reflections
While initially CI activity within NHSBT was very much ‘by the book’, over time there has been more ‘blending’ of the concepts with existing NHSBT processes of controlling and documenting change. This ‘blending’ of CI and quality systems within organisational practice is assisting in positive, ongoing culture change.
In support continued CI ‘super-user groups’ have been created within the organisation, by which each department/stakeholder has a nominated spokesperson for each value stream who meet periodically to update and discuss local CI activity, reaffirm agreed standard work nationally and feedback locally.
In my opinion, without the described improvements and visible evidence of a shift in culture/attitude towards CI, the chimerism service would either no longer be financially sustainable or clinically relevant. I hope that this brief report may be of use or offer reassurance to those implementing or supporting a CI ethos by showing that big changes can be made with small manageable steps.